Raman Spectroscopy

Raman spectroscopy is a scientific technique that is used to study the vibrational, rotational, and other low-frequency modes in a system. It is a powerful analytical tool that can provide information about the structure and composition of a material, as well as its chemical and physical properties.
The basic principle behind Raman spectroscopy is the Raman effect, which is the inelastic scattering of light by a molecule. When light is incident on a molecule, it can be absorbed, transmitted, or scattered. The absorption of light leads to an electronic transition within the molecule, while the transmission of light occurs when the molecule does not interact with the light. Scattering of light, on the other hand, occurs when the molecule interacts with the light in such a way that it changes the frequency or wavelength of the light.
There are two types of scattering that can occur: elastic scattering and inelastic scattering. Elastic scattering occurs when the frequency of the scattered light is the same as the incident light, while inelastic scattering occurs when the frequency of the scattered light is different from the incident light. The Raman effect is an example of inelastic scattering, where the frequency of the scattered light is lower than the incident light.

The Raman effect is a result of the interaction between light and the vibrational and rotational modes of a molecule. When light is incident on a molecule, it can excite the vibrational and rotational modes of the molecule. The energy absorbed by the molecule is then re-emitted as scattered light, with a frequency that is lower than the incident light. This shift in frequency is known as the Raman shift, and it is unique to each molecule, making Raman spectroscopy a powerful tool for identifying and analyzing different substances.
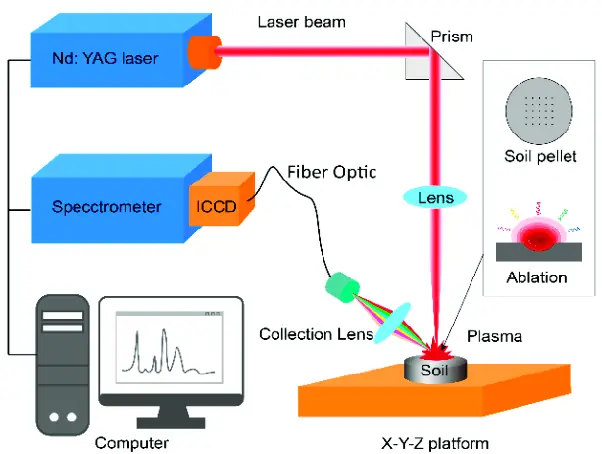
To perform a Raman spectroscopy experiment, a laser is used to excite the vibrational and rotational modes of the molecule. The scattered light is then collected and passed through a spectrometer, which separates the light into its individual wavelengths. The intensity of the scattered light at each wavelength is then measured, and this information is used to create a Raman spectrum, which is a plot of the intensity of the scattered light as a function of the Raman shift.
The information contained in a Raman spectrum can be used to identify the molecular structure and composition of a substance, as well as to study its chemical and physical properties. Raman spectroscopy is a non-destructive technique, meaning it does not alter the sample being studied, making it a valuable tool for a wide range of applications, including pharmaceuticals, materials science, and environmental monitoring.