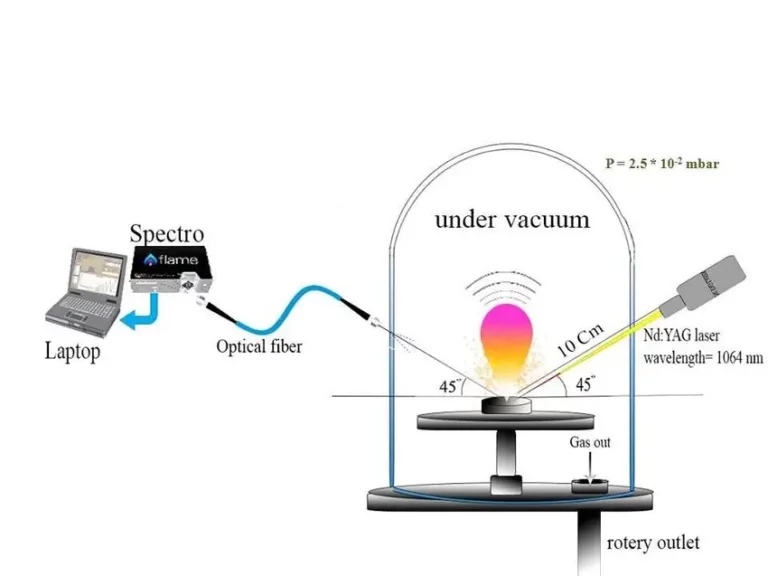
Ultraviolet Fluorescence with 405nm Lasers
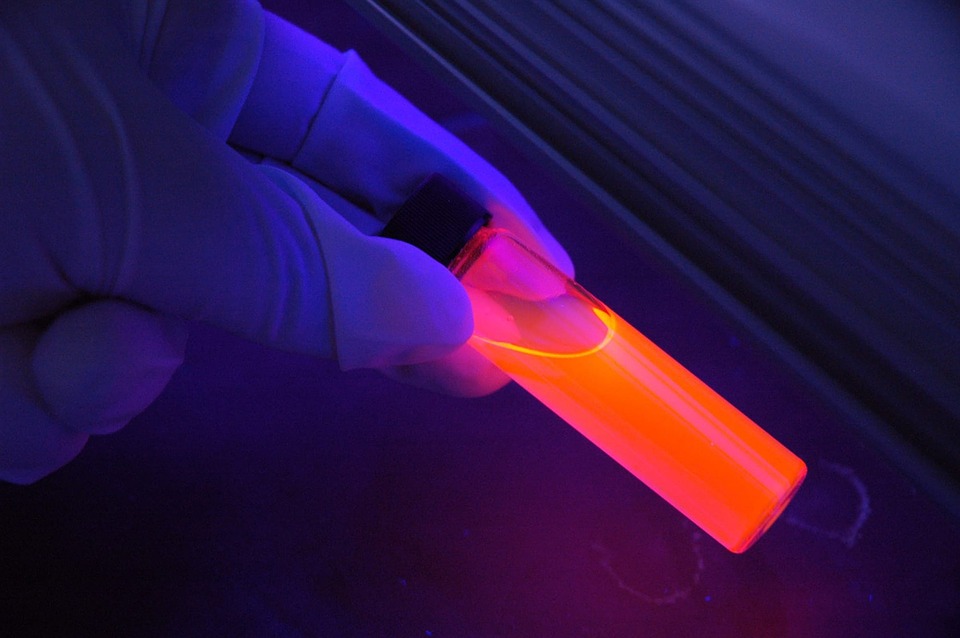
Fluorescence is a type of luminescence, or light emission, that occurs when electrons in certain materials absorb energy from ultraviolet (UV) light or other forms of high-energy electromagnetic radiation and then re-emit that energy as visible light. The word “fluorescence” is derived from the mineral fluorite, which was the first material found to exhibit this phenomenon.
405nm lasers are a type of near ultraviolet (UV) laser that can be used to excite fluorescence in a wide range of molecules. When this type of laser light is shone on certain materials, they will emit light at a different wavelength in a process known as fluorescence. This can be used for a variety of purposes, such as identifying minerals and detecting counterfeit money.

Types of UV Light
There are three types of ultraviolet light: UVA, UVB, and UVC.
UVA light makes up about 95% of the UV light that reaches the Earth’s surface. It is not as harmful as UVB and UVC light, but it can still cause skin damage and cancer.
UVB light is the main cause of sunburn. It makes up about 5% of the UV light that reaches the Earth’s surface.
UVC light is the most dangerous type of UV light. It is completely absorbed by the Earth’s atmosphere and does not reach the surface.
The near ultraviolet range is the part of the ultraviolet spectrum that is closest to visible light and includes 405nm lasers. It is not considered harmful to the skin, but it can cause eye damage without proper safety goggles.
Mineralogy
In the dark, some rocks and minerals glow with an otherworldly light. This eerie, beautiful phenomenon is called fluorescence, and it has captured the imaginations of people for centuries.
Another common use for 405nm lasers is in mineralogy. When shone on rocks and minerals, these lasers can cause them to fluoresce, which can help to identify them. Additionally, the light from these lasers can help to reveal hidden features of minerals that would otherwise be difficult to see.
When UV light hits a fluorescent mineral, it causes the electrons in the mineral to become excited. These excited electrons then emit visible light as they return to their normal state. The color of this light depends on the type of mineral. For example, fluorescent calcite is often yellowish-green, while fluorescent quartz can be red, orange, or yellow.

UV light can also be used to detect certain inclusions in minerals. For example, many diamonds will fluoresce green when exposed to short-wave UV light, due to the presence of trace amounts of nitrogen in the diamond. Other minerals can also be tested for the presence of inclusions using UV light.
UV light can also be used to examine the surface of minerals. Under UV light, many minerals will show up as different colors than they do in visible light. This can be used to help identify minerals, as well as to examine surface features such as scratches or imperfections.