Which color of light has the shortest wavelength?
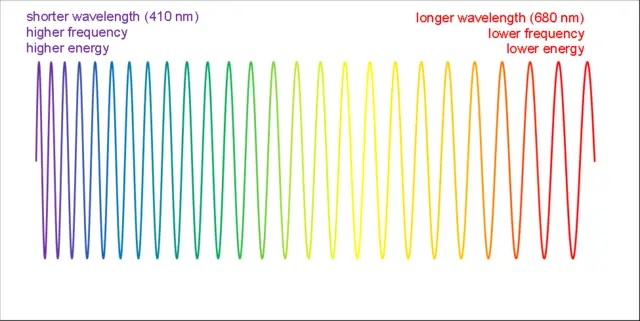
As we explore the vast electromagnetic spectrum, one question often comes to mind: which color of light has the shortest wavelength? The visible light spectrum is the portion of the electromagnetic spectrum that can be seen by the human eye, and it consists of a range of colors, each corresponding to a different wavelength. The color with the shortest wavelength is violet. Violet light has wavelengths ranging from approximately 380 to 450 nanometers. As you move from violet to other colors in the visible spectrum, such as indigo, blue, green, yellow, orange, and finally red, the wavelengths of these colors progressively increase. In contrast to violet, red has the longest wavelength, which ranges from approximately 620 to 750 nanometers.

It is worth noting that colors beyond the visible light spectrum also exist. Beyond the violet end of the spectrum lies ultraviolet (UV) light, which has even shorter wavelengths than violet light, but is not visible to the human eye. Similarly, beyond the red end of the spectrum lies infrared (IR) light, which has longer wavelengths than red light but is also not visible to the human eye. These invisible wavelengths play essential roles in various applications, such as communications, medical imaging, and astronomy.
Fundamentals of Light and Color
Light is a manifestation of electromagnetic radiation, which propagates through space in the form of waves. The visible light spectrum, a small segment of the electromagnetic spectrum, is responsible for generating the colors that our eyes can perceive. Each color corresponds to a particular range of wavelengths, which in turn dictate how the human eye and brain interpret the light.
It’s essential to understand that the visible light spectrum represents only a fraction of the full electromagnetic spectrum, which extends from low-energy, long-wavelength radio waves to high-energy, short-wavelength gamma rays. This broader perspective helps put the significance of visible light wavelengths and their relationship to color into context.
Wavelengths and the Visible Light Spectrum
The visible light spectrum encompasses wavelengths that range from approximately 380 nanometers (nm) to 750 nm. Within this span, each color is associated with a specific wavelength, and colors smoothly transition from one to another in a continuous gradient. At one end of the spectrum, red light exhibits the longest wavelength, followed by orange, yellow, green, and blue. At the opposite end, violet light has the shortest wavelength.
The different colors of visible light are also associated with varying levels of energy. As the wavelength decreases, the frequency and energy of the light increase. Consequently, violet light carries more energy than blue light, which in turn carries more energy than green light, and so on. This relationship between wavelength, frequency, and energy is a fundamental concept in understanding the behavior and interaction of light with matter.

Additionally, the visible light spectrum is only a small part of the broader electromagnetic spectrum, which includes other forms of electromagnetic radiation such as infrared, ultraviolet, X-rays, and gamma rays. These forms of radiation have wavelengths outside the visible range and, therefore, are not perceivable by the human eye. However, they play essential roles in various scientific, medical, and technological applications. Understanding the visible light spectrum is a stepping stone to appreciating the vast and diverse world of the electromagnetic spectrum.
The Color Of Light with the Shortest Wavelength: Violet
Violet light exhibits the shortest wavelength within the visible spectrum, ranging between 380 nm and 450 nm. Due to its short wavelength, violet light has a higher frequency and carries more energy compared to other colors in the visible spectrum. The perception of violet light can be influenced by factors such as the sensitivity of the human eye and the presence of other colors in the environment.

Applications and Significance of Short-wavelength Light
Moving beyond the visible spectrum, ultraviolet (UV) light has even shorter wavelengths than violet light. UV light possesses unique properties, which make it valuable in various fields of technology and research. Some notable applications of UV light include:
- Disinfection and sterilization: UV light can effectively kill microorganisms, making it useful for water treatment, air purification, and surface disinfection in healthcare settings.
- Forensic analysis: UV light can reveal hidden details and substances, such as fingerprints or bloodstains, that are not visible under normal lighting conditions.
- Phototherapy: Controlled exposure to specific UV wavelengths can treat certain skin conditions, such as psoriasis and vitiligo.
However, it’s essential to understand the potential health effects of UV exposure. Prolonged or intense exposure to UV light can cause skin damage, eye irritation, and increase the risk of skin cancer. As such, it’s crucial to take necessary precautions when working with or around UV light sources.
Conclusion
In summary, violet light has the shortest wavelength of any color within the visible spectrum. Understanding the relationship between wavelengths and color perception is essential for various applications and research fields, particularly those involving short-wavelength light. As we continue to explore the electromagnetic spectrum and uncover its potential uses, the importance of light’s unique properties becomes increasingly evident.