What is the longest wavelength light capable of ionizing a hydrogen atom in the ground state?

Ionization is a fundamental process in physics and chemistry, involving the transfer of electrons from an atom or molecule to produce ions. One way ionization occurs is through the absorption of light. In this article, we will investigate the longest wavelength light capable of ionizing a hydrogen atom in its ground state, delving into the intricate relationship between light and atomic structure.
The Hydrogen Atom
Hydrogen, the most basic and abundant element in the universe, serves as the cornerstone for understanding atomic structure. Its simplicity, consisting of just one proton and one electron, makes it an ideal candidate for studying atomic properties. The energy levels of hydrogen atoms are of particular interest, as they directly impact the ionization process. In its ground state, the electron of a hydrogen atom occupies the lowest available energy level, setting the stage for ionization phenomena.
Wavelength, Frequency, and Energy of Light
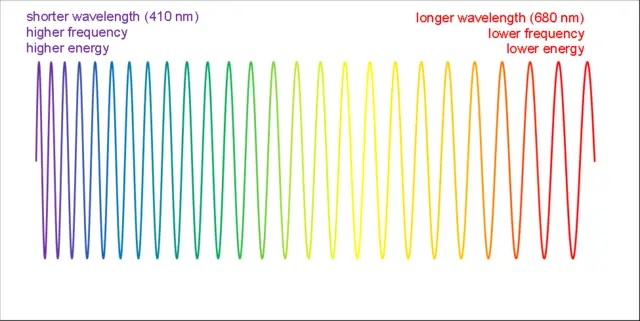
The electromagnetic spectrum encompasses a wide range of wavelengths, from radio waves to gamma rays. Within this spectrum, light exhibits a direct relationship between wavelength, frequency, and energy. The energy of a photon, the elementary particle of light, can be calculated using Planck’s constant and the equation E = h * f, where E is the photon energy, h is Planck’s constant, and f is the frequency of the light.
Ionization Threshold of Hydrogen
To ionize a hydrogen atom, sufficient energy must be provided to overcome its ionization energy, which is the minimum energy required to remove the electron from the atom. For a hydrogen atom in its ground state, the ionization energy is 13.6 electron volts (eV). To calculate the longest wavelength light capable of ionizing hydrogen, we can use the photon energy equation and the relationship between wavelength, frequency, and energy. The result is a wavelength of approximately 91.2 nanometers (nm), which lies in the extreme ultraviolet range of the electromagnetic spectrum.
Applications and Implications
Understanding the ionization threshold of hydrogen has far-reaching implications in astrophysics and astronomy. For instance, the study of interstellar gas and spectral analysis of stars rely heavily on our knowledge of ionization processes. Additionally, lasers play an increasingly prominent role in ionization, with applications spanning across research and industry. As our understanding of ionization progresses, we can expect further developments in laser technology and the myriad fields it influences.
Conclusion
In summary, the longest wavelength light capable of ionizing a hydrogen atom in its ground state is approximately 91.2 nm. This knowledge is crucial for understanding ionization processes, as well as advancing laser technology and its applications. With ongoing research and innovation, we can anticipate exciting discoveries and advancements in the fields of physics, chemistry, and beyond.