If the wavelength of a photon is doubled, what happens to its energy?
Photons are elementary particles that make up light and other forms of electromagnetic radiation. They are unique in that they have both wave-like and particle-like properties. One of the most important properties of photons is their energy, which is closely related to their wavelength. In this article, we will explore the relationship between the wavelength of a photon and its energy, and find out what happens to a photon’s energy if its wavelength is doubled.
The Relationship Between Wavelength and Energy
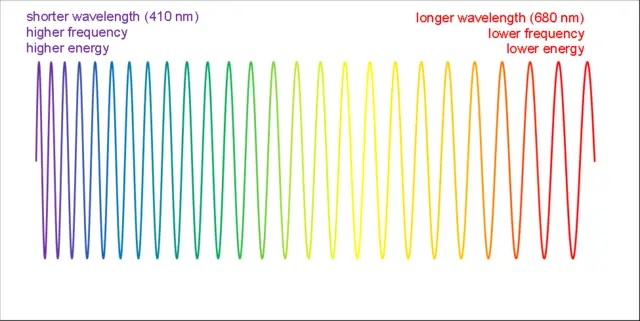
The electromagnetic spectrum is a range of frequencies or wavelengths of electromagnetic radiation, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. The wavelength of a photon is the distance between two consecutive peaks or troughs in the wave. The energy of a photon is directly proportional to its frequency and inversely proportional to its wavelength. The shorter the wavelength, the higher the frequency, and the more energy the photon has. The longer the wavelength, the lower the frequency, and the less energy the photon has. This relationship is described by the equation E = hν = hc/λ, where E is energy, h is Planck’s constant, ν is frequency, c is the speed of light, and λ is wavelength.
The Effect of Doubling the Wavelength of a Photon on Its Energy
When the wavelength of a photon is doubled, its energy is halved. For example, a photon with a wavelength of 400 nanometers (nm), which is in the violet-blue part of the visible spectrum, has an energy of about 3.10 electronvolts (eV). If the wavelength is doubled to 800 nm, which is in near the infrared part of the spectrum, the energy is halved to about 1.55 eV. This means that the photon has less energy to transfer to other particles or to cause ionization.
The Impact of Energy on Photons
The energy of photons is important because it affects their behavior. High-energy photons, such as X-rays and gamma rays, can ionize atoms and molecules, break chemical bonds, and cause damage to biological tissues. Lower-energy photons, such as visible light and radio waves, do not have enough energy to cause ionization or chemical reactions, but they can still be absorbed or scattered by matter. The energy of photons is also important in fields such as astronomy, where it is used to study the composition and temperature of celestial objects.
Conclusion
In conclusion, the wavelength of a photon and its energy are closely related, with energy being directly proportional to frequency and inversely proportional to wavelength. If the wavelength of a photon is doubled, its energy is halved. This has important implications for the behavior of photons and their impact on matter.